

We are converting from volume to mass, so we use density as the conversion factor.

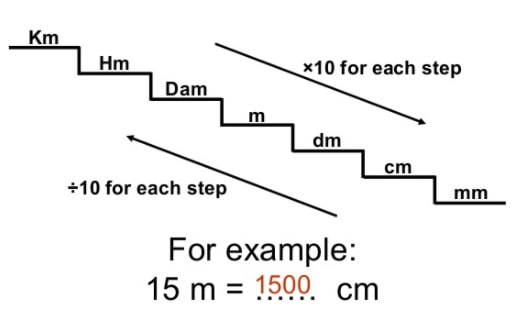
Well, that pretty simple, but at this point I'd like to show the flowchart and how to use it: Click Here for Print Version: Wow! That's confusing! But look at the red density conversion on the flowchart above. Note: make sure that you have the right units where they belong. We need to look up the density of mercury, 13.5 g/mL. Convert 45.9 mL of liquid mercury (Hg) to grams of mercury. Let's first look at a simple example that is likely familiar to you and doesn't involve chemical reactions.Ĭonverting from mass to volume and volume to mass: Density is our conversion factor. To answer such questions involving chemical reactions we always need the balanced chemical reaction that refers to the specific situation. For example, if I start with 10 gallons of gasoline, how many liters of oxygen gas would be required to burn the 10 gallons of gasoline? Or maybe a question such as if I have 10 grams of sugar and 10 grams of sulfuric acid, which one will be used up first (which is the 'limiting reactant'). Grams To Atoms Calculator - Check Grams To Atoms Calculator Online Only on Flowchart & Chemical Conversions Stoichiometry Flowchart & Chemical Conversions According to Wikipedia stoichiometry is the calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions.
We couldn't find a conversion between atoms and grams Quickly convert atoms into grams-force (atoms to grams) using the online calculator for. Given below are some of the problems which are solved step by step based on conversion of grams to atoms. How do you convert grams to atoms? You convert grams into atomic mass units by measureing the size of the gram and multiplying the size by the mass of E=mc2.


 0 kommentar(er)
0 kommentar(er)
